Definition of responders3
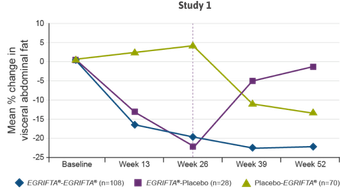
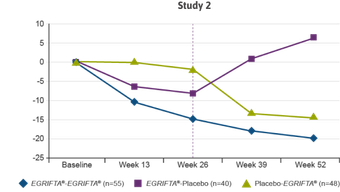
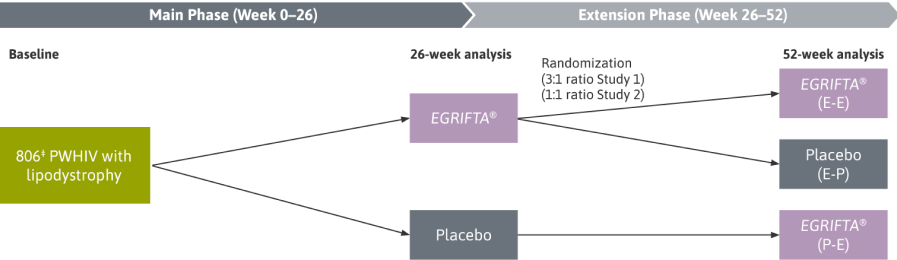
In this post‑hoc analysis, responders were determined independently at Weeks 26 and 52.
- A per‑protocol analysis of adherent patients (>80% compliance with daily EGRIFTA® injections) was used to analyze the clinical effects of EGRIFTA®
- The per‑protocol population included patients who had no major protocol violations and underwent ≥1 post‑dose abdominal CT scan for excess visceral abdominal fat measurement
- As specified a priori in the data analysis plan, a decrease of ≥8% was used to define “responders”2
The results of the post‑hoc responder analysis were not part of the NDA, and therefore were not reviewed by the FDA to support the approval of EGRIFTA®.
The safety and effectiveness of EGRIFTA SV® has been established based on adequate and well‑controlled studies with EGRIFTA® (tesamorelin for injection).
EGRIFTA SV® is not indicated for weight loss management.
EGRIFTA SV® is not approved for use in clinical conditions other than the reduction of excess abdominal fat.

 toll‑free at 1‑833‑23THERA (1‑833‑238-4372).To report suspected adverse reactions, contact
toll‑free at 1‑833‑23THERA (1‑833‑238-4372).To report suspected adverse reactions, contact